Development services
Our development activities are related to two different scenarios:
1
We start from scratch. This means, we perform for a given (existing) molecule (or NME) formulation studies, develop a freeze drying recipe, optimize the recipe using innovative PAT and QbD principles, have an eye on the transferability of the cycle to larger scale. In parallel, we apply an analytical orchestra to determine physicochemical properties of the liquid formulation and final drug product, conduct stability testing at Ich conditions, etc.
In the course of our development approach, we reduce the development effort to a minimum, following a straight development paradigm and avoid unnecessary experiments. Moreover, freeze drying cycle development at GILYOS focuses on all three phases of the cycle, not only on primary drying.
2
We start with an existing cycle. Typically, this is a manufacturing cycle which requires troubleshooting or optimization. Our innovative equipment and expertise allows us to scale such cycles down and to further investigate the problem or look for optimization potentials.
We are scientists, dedicated idealists working on the best solution for your product.
Some development services key facts:
- Gilyos has a successful track record to develop formulations and processes for small molecules, proteins, peptides, vaccines, live bacteria, nanoparticles and other complex (drug delivery) systems.
- Gilyos has the permission to work with biological agents up to and including Biological Safety Level (BSL) 2, including human and animal pathogens, and GMOs (Genetically Modified Organisms). We ensure process development under “real” conditions, i. e. without additional containment solutions which impairs the applicable process conditions and scalability / transfer.
- Gilyos has substantial knowledge in thermal characterization of liquid and solid products using, for example, MDSC, LT-FDM, and other innovative techniques.
- Gilyos has substantial knowledge in application of crucial analytical procedures used in freeze drying (XRPD, Karl Fischer residual moisture, DVS, SEM, BET, and many more).
- Gilyos can help to identify an optimum primary packaging material (vial, syringe, etc.) and has strong expertise in performance testing of such materials.
- Gilyos has stability chambers to conduct various stability testing scenarios at ICH conditions.
- Gilyos performs freeze drying cycle design and optimization (new and existing products) using state of the art Process Analytical Technology (PAT), controlled nucleation and QbD concepts.
- Gilyos has expertise in mechanical testing of primary and secondary packaging materials, e. g. piercing performance of stoppers, or sealing performance of blister packaging.
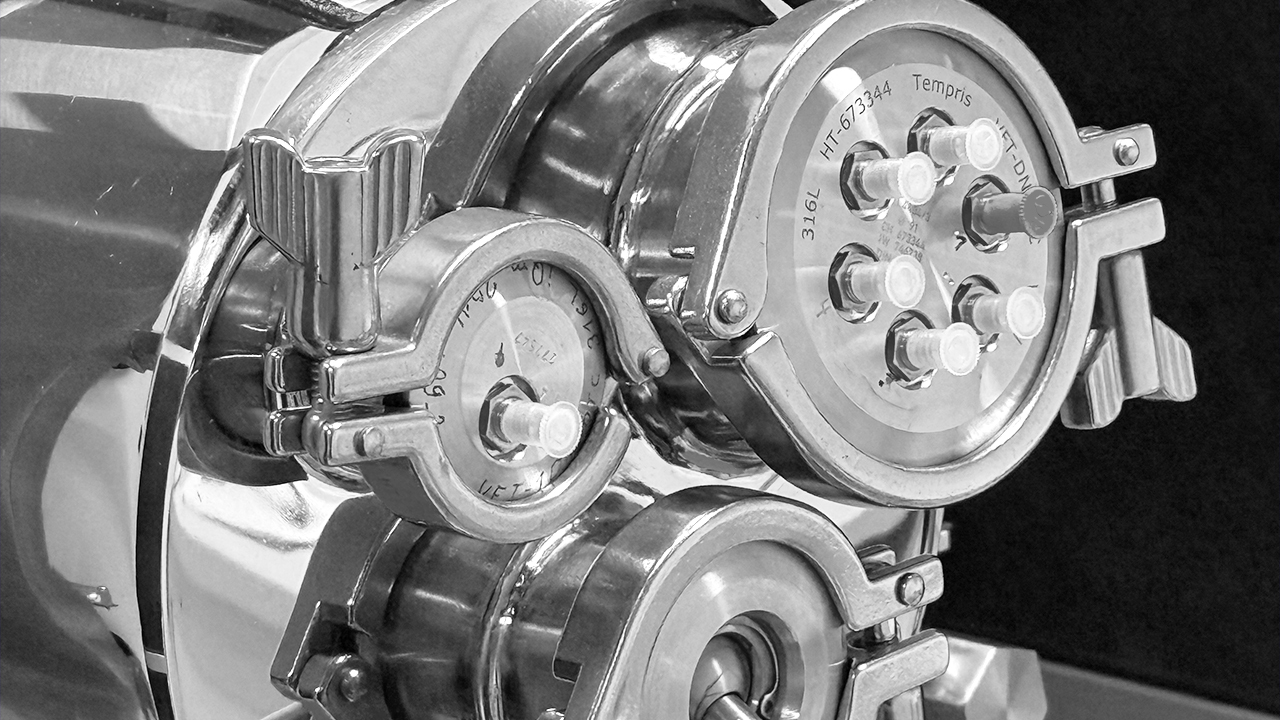
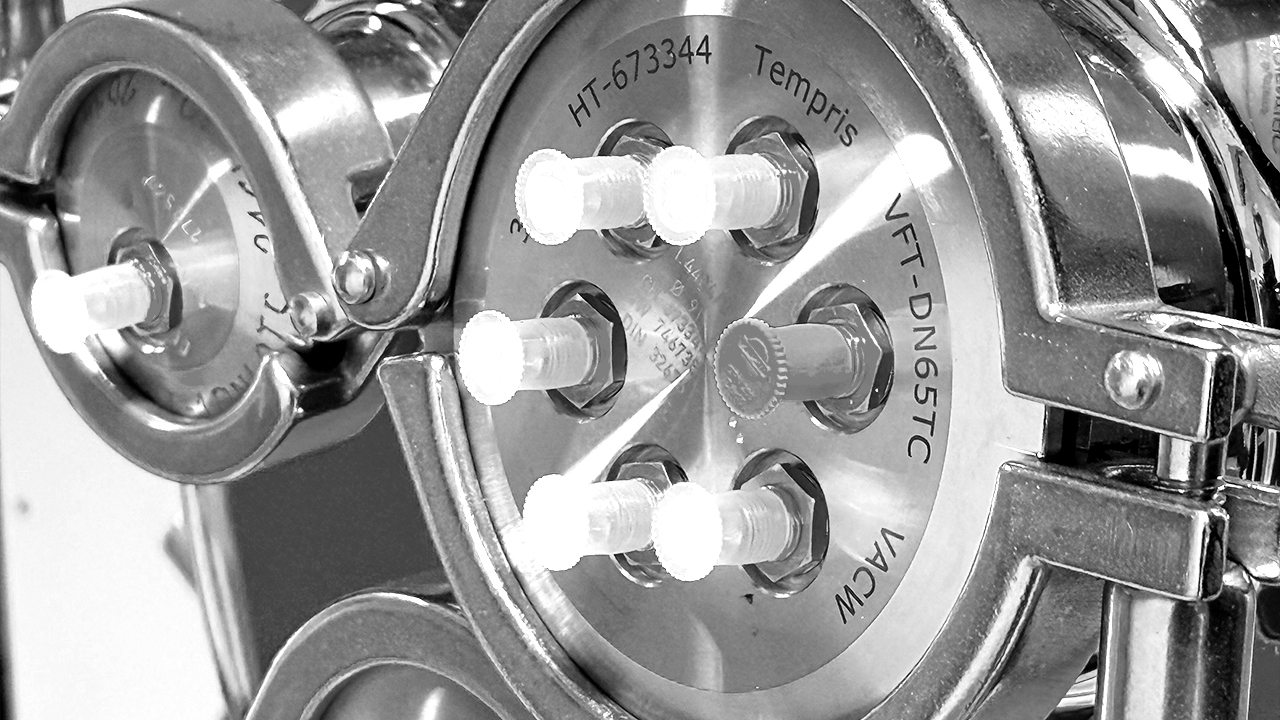

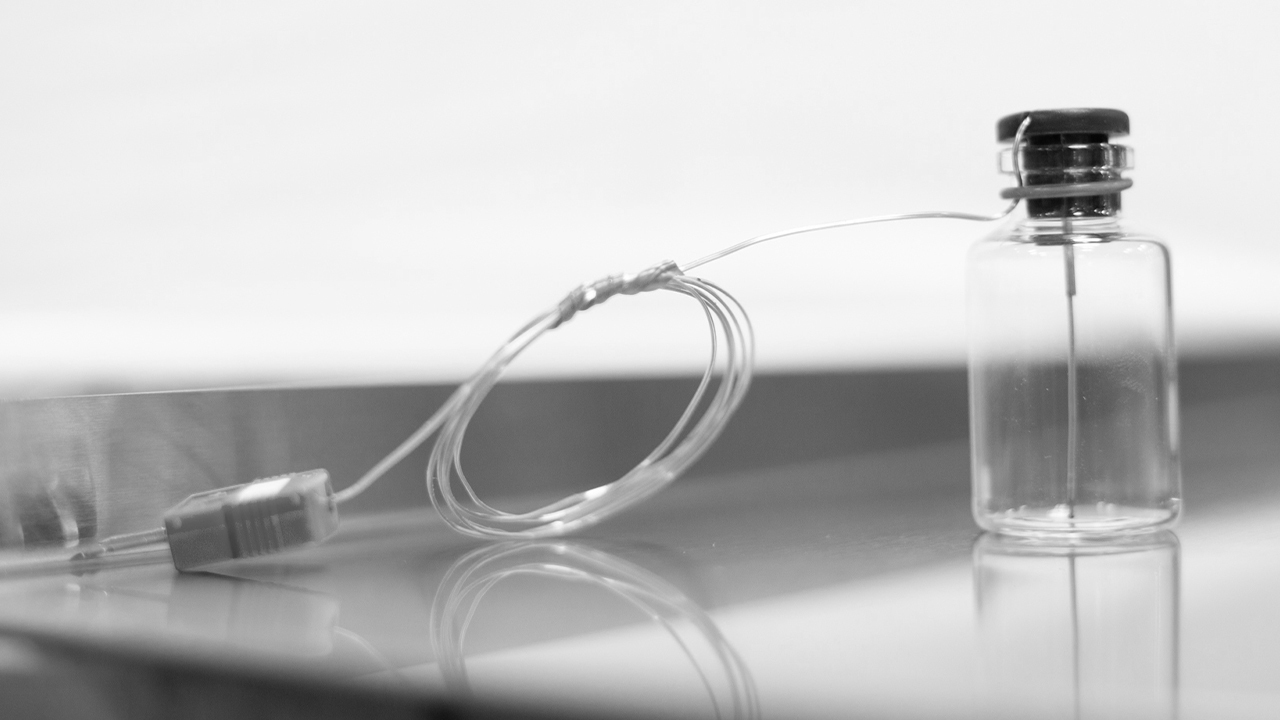


We use the most recent, highly innovative process equipment, top notch analytical tools and instrumentation for freeze drying development services. Among others, our pilot scale LyoStar™ freeze dryers (0.5 m² total shelf area) are equipped with MTM / Smart™ Freeze Dryer technology, TDLAS / Smart™ Freeze Dryer technology, ControLyo™ controlled nucleation and TEMPRIS™. We also use proprietary tools which are not (yet) available on the market.
Important statement from our side: We apply such instrumentation, we don’t want to sell it to you! Equipment sales is not our business.
Faqs
Can Gilyos handle formulations containing co-solvents?
Yes. We have freeze drying equipment specifically designed to process formulations containing co-solvents.
We have a bulk freeze drying application which requires process optimization. Can Gilyos support us?
Yes. We have a long history of development activities targeting bulk freeze drying, starting from stainless steel trays, plastic trays and GORE LyoGuard™ trays.
We want to determine Kv of our vials. Is this something Gilyos can practically do for us?
Yes and no. We have determined Kv of various vial types and sizes over the past years, supported development of primary packaging materials. We published a number of research papers in this regard. It is, however, important to understand that any Kv determination is somewhat dependent on the freeze dryer scale. Kv is not a physical constant, not a single value, but dependent on the heat transfer situation in a chamber and corresponding heterogeneity.
Is Gilyos following a QbD approach during development?
Yes. However, QbD and Design Space definitions are more time consuming and require more experiments. And this approach is only useful when data of the laboratory or pilot scale freeze dryer AND production unit are available. Dr. Henning Gieseler, CSO of Gilyos, is one of the pioneers in the field of QbD in freeze drying. Please have a look at the Gilyos LyoLibrary for more information.